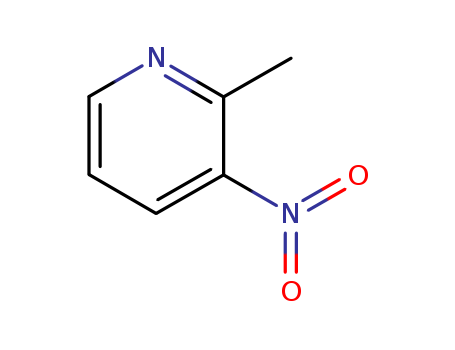
2-Methyl-3-nitropyridine CAS NO.18699-87-1
- FOB Price: USD: 1.00-1.00 /Kilogram Get Latest Price
- Min.Order: 1 Kilogram
- Payment Terms: L/C|D/P|T/T|Westem Union|
- Product Details
Keywords
Quick Details
- ProName: 2-Methyl-3-nitropyridine
- CasNo: 18699-87-1
- Molecular Formula: C6H6N2O2
- Appearance: Brown powder
- DeliveryTime: 5 day
- PackAge: 25kg/plastic drum,200Kg/iron drum
- Port: Dongguan
- ProductionCapacity: 100 Metric Ton/Month
- Purity: 98%~99%
- Storage: sealed storage in shady and cool wareh...
- Transportation: by air
- LimitNum: 1 Kilogram
Superiority
1. Introduction of2-Methyl-3-nitropyridine The 2-Methyl-3-nitropyridine, with its CAS NO18699-87-1, is a kind ofBrown powder. I…
Details
1. Introduction of 2-Methyl-3-nitropyridine The 2-Methyl-3-nitropyridine, with its CAS NO 18699-87-1, is a kind of Brown powder. It has synonyms of 3-Nitro-2-methylpyridine;3-Nitro-2-picoline;NSC 311455;2-Picoline,3-nitro- (7CI,8CI);2-Methyl-3-nitropyridine. 2. Properties of 2-Methyl-3-nitropyridine (1) XLogP3-AA 1.1 (2) H-Bond Acceptor 3 (3) Exact Mass 138.042927 3. Structure descriptors of 2-Methyl-3-nitropyridine IUPAC Name: 2-methyl-3-nitropyridine InChI: InChI=1S/C6H6N2O2/c1-5-6(8(9)10)3-2-4-7-5/h2-4H,1H3InChIKey: CCFGTKQIRWHYTB-UHFFFAOYSA-N Canonical SMILES : CC1=C(C=CC=N1)[N+](=O)[O-] 4. Synthesis of 2-Methyl-3-nitropyridine
(4) MonoIsotopic Mass 138.042927 (5) Topological Polar Surface Area 58.7
(6) Heavy Atom Count 10 (7) Complexity 132 (8) Covalently-Bonded Unit Count 1
(9) Feature 3D Acceptor Count 3 (10) Feature 3D Anion Count 1
(11) Feature 3D Ring Count 1 (12) Effective Rotor Count 1
(13) Conformer Sampling RMSD 0.4 (14) CID Conformer Count 1
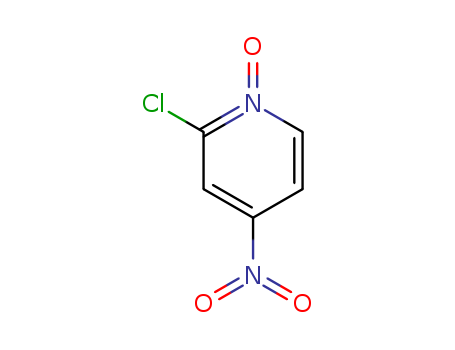
The title compound has been obtained by several related ways: The reaction of 2-chloro-3-nitropyridine (I) with methylboronic acid by means of Pd(PPh3)4 and K2CO3 in hot dioxane gives 2-methyl-3-nitropyridine (III), which is condensed with dimethylformamide dimethylacetal (IV) to yield 2-[2-(dimethylamino)vinyl]-3-nitropyridine (V). The oxidation of (V) by means of NaIO4 affords 3-nitropyridine-2-carbaldehyde (VI), which is condensed with semicarbazide (VII) to provide the corresponding semicarbazone (VIII). Finally, the nitro group of (VIII) is reduced with SnCl2 or Na2S to furnish the target 3-aminopyridine-2-carbaldehyde semicarbazone. 2-Methyl-3-nitropyridine (III) can also be obtained by condensation of 2-chloro-3-nitropyridine (I) with diethyl malonate (II) by means of Na, followed by decarboxylative hydrolysis with H2SO4 at 125 C. The direct oxidation of 2-methyl-3-nitropyridine (III) with SeO2 in dioxane gives carbaldehyde (VI), which is treated with ethyleneglycol (IX) and Ts-OH to yield the cyclic acetal (X). The reduction of (X) with H2 over Pd/C in ethanol affords 3-aminopyridine-2-carbaldehyde ethylene ketal (XI), which is treated with semicarbazide (VI) and HCl to afford the target 3-aminopyridine-2-carbaldehyde semicarbazone. The condensation of 2-chloro-3-nitropyridine (I) with tributyl vinyl tin (XII) Pd(PPh3)4 and PPH3 in refluxing toluene gives 3-nitro-2-vinylpyridine (XIII), which is oxidized with O3 and M






![Bis[3-(triethoxysilyl)propyl]tetrasulfide](http://file1.lookchem.com/cas/reactions/2021/05/25/2062235.png)
![Bis[3-(triethoxysilyl)propyl]amine](http://file1.lookchem.com/cas/reactions/2021/08/11/1802769.png)